 |
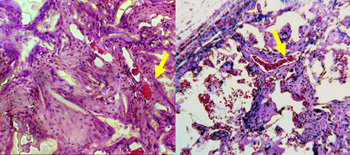
Figure 1. Vascularization of the implanted scaffolds. Left: poly(DTE carbonate), right: poly(DTE-co-10% DT carbonate) with 8% PEG crosslink. Yellow arrow indicates "fingers" of blood vessels leading tissue growth into the scaffold.
|
Inflammation of wounds is an important and complex aspect of the immune system, which provides challenges for biomaterials researchers. Shortly after a wound is inflicted, the process of inflammation begins as resident macrophages and dendritic cells sense foreign antigens. These cells initiate an immediate immune response by secreting pro-inflammatory compounds, such as Tumor Necrosis Factor Alpha and reactive oxygen species (ROS), to kill any foreign cells, such as bacteria, that have infiltrated the wound site. This process needs to be controlled spatially and temporally so as to not cause undue damage to native tissue. A second function of inflammatory cells is to stimulate tissue repair. These same cells do so by secreting pro-survival and pro-angiogenic factors, such as Vascular Endothelial Growth Factor (VegF), into their environment to recruit the cell types that will re-populate the wound bed.
Therefore, the research goal is to minimize the number of pro-inflammatory macrophages while trying to maximize macrophage stimulation of angiogenesis and repair, since in the context of biomaterial implantation, the pro-inflammatory functions of macrophages and dendritic cells are less desirable. The Kohn Lab's research achieves this balance by incorporating bioactive molecules (peptides, small molecule inhibitors, redox compounds) into biomaterials with repair oriented scaffolds that will influence the ability of macrophages to sense and respond to a biomaterial with a repair oriented inflammatory response.
Project Leader: Jared Bushman, PhD
Funding Source: National Institutes of Health (R21HL091465)

